Brains of Britain: Behind the scenes of the war on cancer
When you give money to cancer research charities, do you know what difference it makes?

When lab-based cancer experts spend countless hours gazing into microscopes, what are they looking at? And as more time passes, how much closer are they to finding a cure for this cruel illness?
Weekend went on a mission to find out. And we didn't have to travel far to do it, because it turns out that one of the UK's leading brain cancer research scientists heads her team from right in the heart of Wolverhampton.
Dr Tracy Warr, 51, is one of a very small group of researchers at the Neuro-Oncology Research Centre, based in the University of Wolverhampton. They work tirelessly to find ways to help extend the quality and length of the lives of patients with brain tumours.
We put on our white lab coats to join Tracy and her team in the centre to find out more about what they're working on. We're amazed by the cutting-edge scientific exploration that's happening right here on our doorstep.
But before we find out about the work they're doing to fight it, first of all, we had to understand a bit more about the illness.
Overall in England and Wales, for all types of cancerous (malignant) brain tumours in adults, only 40 per cent of people survive for one year or more after they are diagnosed. It's a miserable outlook, and one that the team here want to change. Tracy makes it clear to us that there's unlikely to be one huge 'eureka!' moment in a cancer research lab when it comes to finding a cure, though.
"There's never going to be a cure for cancer because there are so many different types of the disease," Tracy says. "There are more than 120 different types of brain tumour and they've all got different genetic aberrations, which means they'll respond differently to drugs and different treatments. So there's never going to be a cure."
But that doesn't mean that the team aren't going to work solidly to add to the 'puzzle' by finding different pieces of it through research. Tracy explains: "There may be a drug that works in several different types of cancer, or sub-sets of patients, and we need to look at that instead of a cure. We must make a series of small steps to treat patients better. To increase their life expectancy and the quality of their life, which should never be downplayed, in small incremental steps.
"A refinement of a technique or a drug may only make a few months difference, or a few weeks, but when you add it up to the other refinements that preceded it and other changes that can be brought in in the future, then over a long period of time you can see that you've increased the survival of this cancer from six months to five years."
Tracy tells us how one of her PhD students, Prasanna Channathodiyil, has come close to a huge breakthrough this year: "Anna graduated in May. She's probably got us the closest we've ever been to getting a drug into patients than we've ever been in my career. She found a deficit in a pathway that makes proteins in tumour cells. So we're collaborating with a group at Imperial College as there's a trial drug being used in liver cancer that hasn't been trialled yet in brian tumours. Because it's a drug that's already licenced, it's a lot quicker to get that out to patients than having to make a chemical structure and go through the process of getting that out. We predict it'll be a maximum of 25 or 30 per cent of patients that it'll help, but it's better than we've got now!"
Making use of other drugs that already exist is something that Tracy tells us is crucial. "The other option to repurposing drugs is to create new ones. That takes years – 10 or 15 years – and around
US$1 billion to get that into patients. And that funding isn't available."
She tells us about her career so far.
"I took a very traditional path. I did biology, chemistry and maths A-Levels and I did a first degree in genetics at Leeds University and then a PhD in genetic toxicology looking at how environmental chemicals may stimulate cancer and be the cause of it. I then moved to London and did a first postdoc looking at a childhood muscle tumour and then I got the option to join the Queen Square Institute of Neurology (a world-class facility based at the University College London). That was in 1993.
"I always wanted to be a research scientist. I think from my early teens, when I was making positive career decisions rather than 'I want to be an archaeologist' which was my ten-year-old self! I enjoyed biology much more than the physical sciences. I always planned to make a difference to health, so it was very much around wanting to do medical research. So from my A-Levels I found that I really enjoyed the genetics side of things which was very much in its infancy at the time. There were only ten universities across the country that did a genetics degree.

"When the first human genome was sequenced it took ten years and so much money you wouldn't believe it. And now you can do it in a day and it costs £500! So the rate of technology in my career, let alone my student days, has changed exponentially. Things move on so quickly.
"I was 27 when I started working in brain tumour research and I knew quite quickly that it was where I was going to spend the rest of my career. There was so much to do and there'd been so little research carried out. The prognosis for patients was so poor and it was such a complex disease. So for me, it was the feeling that I could make a difference to patients in my career, and the knowledge that it was going to be a very interesting journey.
"When I first moved into brain tumour research it was to look at paediatric tumours. There's about 400 new causes of paediatric brain tumour in the UK a year. It doesn't sound very many, and a lot of those – around half – will be benign and can be resected quite successfully. The problem is, if you're going into a child's head and removing a tumour, the surgeon is going to unavoidably cause some neurological damage.
"As a 21-year-old I was very naively optimistic that yes, you can eradicate this illness. But now I realise that even just making a small difference is good. I learned that quite early on in my brain tumour research career. We're quite a small community of researchers in this country, and you get exposed from a very young age just how bad a disease it is. The great breakthroughs that haven't come to fruition is a bit of a reality check.
One common drug that Tracy and the team are looking into repurposing is metformin. It's currently used as a medication for diabetes, and has been in circulation since the 1950s. Cancer cells need a lot more sugar than others, and when fed more glucose, grow more. By what the team describes as an 'accident', it's been found that diabetics who were taking metformin had a lower chance of getting cancer, and the cancers were less severe. Therefore, the research team are spending a lot of time and energy working out whether metformin may be repurposed as a cancer treatment.
To do these tests, the team has to grow cancer cells in flasks and dishes in the lab. They go to Liverpool each week to collect samples of brain tumours from willing donors.

Tracy says: "We need to be able to work on and experiment on tissue. Patients are very altruistic in the main, and they'll donate their tissue to research and they're very glad to do that. We're currently working with a number of hospitals across the country, and we collect the tissue. We then grow those tumour cells in the laboratory. That's fabulous, it's one good thing about working with malignant brain tumours. It's a horrible disease with a really poor outcome, but they grow very well in petri dishes. So if you've got a piece of a tumour, you have a snapshot of what's happening in that tumour, in that patient at the time it was taken out and look at its molecular biology and genetics.
"What you can't do, though, is see how it would respond to any new therapies or anything else you want to do to change its behaviour. By growing them in the lab, we can test different drugs, different drug combinations, new, old and repurposed drugs and we can introduce genes in and silence others and look at their behaviour. But does it stop the cells growing? Does it stop the tumour cells migrating into other cells?"
To show us an example of this in action, Tracy's colleague Dr Farjana Rowther takes us to a microscope. "They're like babies," she tells us about the cancer cells she's growing, "you have to feed them on time and you can't leave them alone."
Farjana, 35, shows us six wells under the microscope, each of which contain cancer cells from the same patient. To the naked eye, nothing can be seen. But close up, we see how she's meticulously separated the cells into six more sections, each of which contains the same number of cells. She was shocked, she tells us, when she came into work this week to find that some of the cells had started to try and move away from the others, splitting into other tumours. This, she shows us, is how cancer spreads in the body. It's chilling to see.
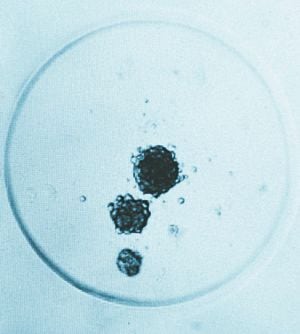
It's now down to Farjana to find out why the cells have done this – especially considering that not every sample in the six sections has done the same thing. It seems an impossible puzzle, but one that Farjana is determined to solve.
Even if the researchers reach a dead end with their sample cells, they keep them cryogenically frozen, just in case. Because of this, they can defrost them further down the line and continue their work. They have around 3,500 cultures in the lab that span a 40-year period. They still use cancer cells from patients that they received back in the 80s.
Keeping a centre like this is, as you can imagine, expensive. And brain tumour research only received one per cent of the funds available for cancer research in the UK. Tracy explains why the money is so desperately needed: "Our research is very expensive because of the cost of people and laboratory consumables. As a ball-park figure, for every member of my team, whether they're a PhD student or a postdoc, to do research properly, it costs about £15,000 per person, per year. And that's just on the chemicals and things that they'll use in the laboratory."

Tracy is keen to remind us that no experiment is ever a failure – it just may not give you the answer you expect. She says: "One of the best things about doing research is that you don't know what is going to come up. Every piece of research that you do, and every paper you publish that goes out into the scientific domain is adding another piece to the puzzle. If we hit dead ends, then it means that others won't go back and repeat that work. It's important from that point. Or it could inspire a fresh pair of eyes to read it and think wow, now the piece of research that I'm working on makes sense. We do that all the time, picking up on other people's work."
One of the biggest scientific discoveries that Tracy's ever identified was one that made it more clear just what a huge task brain tumour researchers have ahead of them. She explains: "Not only do you have the 120 different types of brain tumour, even in tumours that are classified under the same name, that look the same under the microscope, they have different genetic abnormalities."

It seems like an impossible illness to beat. Even one tumour isn't necessarily the same throughout: "The tumours just have so many different ways of going wrong," Tracy says. "Even in the same tumour from a single patient, one area of the tumour may have one genetic fingerprint, and another bit may have another. So if you're targeting a drug against portion one, it might kill off all of that one side, but it'll leave some residual tumour behind which will invariably grow again."
Brain tumours kill more children and adults under the age of 40 than any other cancer
Only one per cent of the national spend on cancer research has been allocated to this devastating disease
Each year, 16,000 people are diagnosed with a primary or secondary brain tumour
Fewer than 20 per cent of those diagnosed with a brain tumour survive beyond five years compared to an average of 50 per cent across all cancers
Brain tumours represent one per cent of cancers diagnosed yet three per cent of cancer deaths
Around 3,600 people a year die from a brain tumour in the UK
One in 50 of all people who die under the age of 60 die from a brain tumour
71 per cent of those that die of a brain tumour are under the age of 75 (compared to 47 per cent for all cancers)
Up to 40 per cent of all cancers eventually spread to the brain
Unlike most cancers, incidences and deaths from brain tumours are increasing
At the current rate of spend on research, it could take 100 years for brain cancer to catch up with developments in other diseases and find a cure
Brain tumours kill more children than leukaemia or any other cancer
With so many different things to research, we wonder where Tracy and the team even begin. She says: "It's really down to the most common type of malignant brain tumour in adults, which probably makes up around 80 per cent of cases and has a very poor outcome. That's where we're pushing our energies."
And to do that, Tracy and the team need funding. "If you have got a low grade benign tumour, then it can be removed surgically successfully. But not malignant brain tumours, because they're insidious and spread through the normal brain.
"We work with a lot of charities and we've hosted open days here at the research centre so that patients and their carers can see what's being done. Sadly, they know that it's not going to benefit them. But patients with a disease like a brain tumour, where the treatment is so ineffective, are really glad to see that something's going to be happening for the future.
"A lot of people have set up small memoriam funds to help us, as they want to give to site-specific research. Less than one per cent of the national cancer research spend is on brain tumour research at present. But brain tumours are the cause of the highest number of life years lost in people under 40 than any other cancer. So people feel that if they give to the big charities, it's going to go to general cancer research that may not benefit brain tumour patients.
"Research funding has got harder and harder to obtain. There's certainly less government funding than there was 20 or 30 years ago, so we rely on donations from families and family trusts and small charities."
Tracy guarantees that any money donated to the centre in Wolverhampton can be spent on exactly the area of research that the donor wishes. It's incredible to see the team in action, and we find it fascinating that the people we meet in the lab are at the frontier of science.
True superheroes don't wear capes, we learn, watching Tracy and her team in action. Sometimes, they wear lab coats instead.
For more information on the research centre, or to obtain a gift aid form to make a donation, contact Tracy at t.warr@wlv.ac.uk
By Kirsty Bosley